28 December 2019
 ‘Reproducing human and cross-species drug toxicities using a Liver-Chip’, delves into the research by Emulate Inc. with Janssen Pharmaceuticals, Astra Zeneca and the Wyss Institute at Harvard on the development of liver chips using species-specific cells to predict drug induced liver toxicity and reduce drug attrition rates. On the same lines of drug induced toxicity, Matsui et al. worked on developing 2D model systems with human iPSCs to predict cancer drug-induced cardiotoxicity, described in their paper ‘Cell-based two-dimensional morphological assessment system to predict cancer drug-induced cardiotoxicity using human induced pluripotent stem cell-derived cardiomyocytes’. Finally for this edition, ‘Human colon-on-a-chip enables continuous in vitro analysis of colon mucus layer accumulation and physiology’ by Sontheimer-Phelps et al. from the Wyss Institute, reports on their model which recapitulates the structure and composition of the native mucus layer of the human intestine – enabling in-depth study of this important defensive barrier.
‘Reproducing human and cross-species drug toxicities using a Liver-Chip’, delves into the research by Emulate Inc. with Janssen Pharmaceuticals, Astra Zeneca and the Wyss Institute at Harvard on the development of liver chips using species-specific cells to predict drug induced liver toxicity and reduce drug attrition rates. On the same lines of drug induced toxicity, Matsui et al. worked on developing 2D model systems with human iPSCs to predict cancer drug-induced cardiotoxicity, described in their paper ‘Cell-based two-dimensional morphological assessment system to predict cancer drug-induced cardiotoxicity using human induced pluripotent stem cell-derived cardiomyocytes’. Finally for this edition, ‘Human colon-on-a-chip enables continuous in vitro analysis of colon mucus layer accumulation and physiology’ by Sontheimer-Phelps et al. from the Wyss Institute, reports on their model which recapitulates the structure and composition of the native mucus layer of the human intestine – enabling in-depth study of this important defensive barrier.
Prediction of species-specific liver toxicity
Drug-induced liver injury (DILI) is a major cause of drug attrition – animal testing shows very low concordance to human data and thus pre-clinical drug testing using animals fails to predict the human response. Selecting drugs with a low likelihood of DILI is a major challenge for the pharmaceutical industry and one which we may be closer to addressing. Ground-breaking research from Emulate Inc. together with Janssen Pharmaceuticals, Astra Zeneca and the Wyss Institute at Harvard has revealed that Organs-on-Chips could be used in predictive toxicity testing – potentially allowing the full replacement of animals in hepatotoxicity safety testing. This extensive research ‘Reproducing human and cross-species drug toxicities using a Liver-Chip’ was published in Science Translational Medicine on November 6 and documents the development and characterization of Liver-Chips created from rat, dog or human cells.
Several different models with increasing cellular complexity were created using Emulate’s functionalized chips. Primary hepatocytes were seeded onto the upper channel; dual cell models incorporated liver sinusoidal endothelial cells in the lower channel whilst the quadruple cell models employed a mixture of liver stellate cells, Kupffer cells and hepatocytes in the upper channel. All chips were created with species-specific cells in order to produce relevant models of dog, rat or human liver. Measurement of metabolic capacity revealed enzyme activities at least equivalent to that of freshly isolated hepatocytes – the gold standard model in pharmaceutical research. The models were then used to evaluate the hepatotoxic effects caused by known toxicants – revealing some very important findings. The first was that the chip models were more sensitive at detecting toxicity than the more ‘traditional’, less dynamic, monoculture models (showing that chips support better hepatic functionality) and the second was the recapitulation of species-specific responses. Data indicated that bosentan – responsible for cholestasis in humans but not rats or dogs – reduced albumin secretion from the human Liver-Chips, with an IC50 equivalent to the plasma level of bosentan associated with DILI clinically. Further investigations using the human Liver-Chips showed that they could be used to link drug mechanism to a functional readout – representing an important feature that could be vital for uncovering the toxicities of potential new drug candidates. Species differences in steatosis and fibrosis in response to known toxicants were apparent in the different Liver-Chips and importantly, treating chips with fialuridine adversely affected the human Liver-Chips, but did not compromise the function of the rat Liver-Chips. Fialuridine was discontinued in phase 2 clinical trials after several incidences of liver failure and many deaths – but preclinical animal tests with fialuridine had not predicted any toxicity. Additionally, here the potential of human liver chips to contradict toxicity seen in animals was demonstrated – again showing the importance of using a human-relevant system with which to predict human responses.
The data presented in this paper indicate not only that using human Liver-Chips can reveal potential toxicities, but also that the chips provide an indication of the mechanism of action, with the multi-cellular models revealing the extent of involvement of different cell types. This paper represents a hugely important step forwards in our ability to predict hepatotoxicities and the human Liver-Chips have immense potential for safety testing, disease modeling, biomarker identification and even the discovery of idiosyncratic liver toxicities prior to clinical trial and as a superior substitute for animal testing.
Prediction of drug induced cardiotoxicity
Drug-induced toxicity is a huge problem for people receiving long-term treatments and one of the main causes for drug attrition in pre-clinical trials in animals. Many drugs are known to cause cardiotoxicity and the problem is a main challenge for cancer patients, to the point that a new field in oncology, called cardio-oncology, has been developed to deal with drug-induced cardiotoxicity in cancer patients.
There are only a few in vitro tests for predicting whether a drug has the potential to be toxic, and lead to heart failure, by causing sustained contractile dysfunction. Currently, the only way to do such test is by using an isolated perfused animal heart, in most cases obtained from a rodent. However, this approach is inefficient, labor intensive, and time-consuming, besides the fact that it cannot be used for high-throughput screening. Also, how the animal heart responds to the potential harmful effects that a drug may have on the human heart may be very different.
Sarcomeres are the main structural components of the myofibrillar fibers and the ones who do all the myocardial contraction and relaxation work. By examining these components, it is possible to evaluate whether or not a given drug is cardiotoxic. In a new study published in the journal Toxicology and Applied Pharmacology, ‘Cell-based two-dimensional morphological assessment system to predict cancer drug-induced cardiotoxicity using human induced pluripotent stem cell-derived cardiomyocytes’, a collaborative team of researchers from Japan and the USA present an efficient and simple, low-cost, 2D morphological assessment assay that works with human induced pluripotent stem cells-derived cardiomyocytes. The group tested 28 drugs of which 16 are used in cancer therapy and are known to cause cardiotoxicity (some examples include doxorubicin, imatinib, and sunitinib) while 12 are used for treating other diseases and are not associated with harmful cardiac effects (used as control).
The system considers two parameters to establish the status of the heart based on sarcomere evaluations and to predict whether a drug may be cytotoxic: the number of cell nuclei and the sarcomere morphology. Based on these parameters, the group created an index for sarcomere health that is the ratio of the number of cells with well-organized sarcomeres to the total number of cells observed in each well. Using the 2DMA (two-dimensional morphological assessment), the group was able to predict the potential cardiotoxicity of the drugs tested with 81% sensitivity and 100% specificity following only 72 hours of exposure. This level of assurance is in some cases higher than that obtained with 3D methods that are either more expensive or time-consuming than the method developed by the group.
Previous systems have been used to evaluate sarcomere structure in vitro, but these techniques are expensive, labor-intensive and time-consuming and thus cannot be used for routine drug tests. An alternative for testing drug toxicity that is straightforward is necessary if we ever want to see the day when each patient will have their own cells tested so the promises of a customized medicine can finally be delivered. The 2DMA developed by the international group is an important step towards a medicine that is precise, feasible, reliable, and efficient while it does not rely on animals to develop and deliver the new tests and treatments so much needed for cancer patients, as well as many others suffering from a myriad of diseases.
Analysing colon mucus layer accumulation and physiology
The most recent paper by Sontheimer-Phelps et al. from the Wyss Institute, ‘Human colon-on-a-chip enables continuous in vitro analysis of colon mucus layer accumulation and physiology’ uses a human-relevant microphysiological system to unravel the mysteries of the intestinal mucus layer. This mucus layer is incredibly important for normal function and for defence of the body against potential pathogens. Defects or breaches in its barrier function can give rise to bacterial entry to the body- creating inflammation that may lead to chronic conditions such as ulcerative colitis and inflammatory bowel disease. However, until now, it has been difficult to access (human) mucus for closer analysis – organoid models may only secrete a small amount – to the inaccessible centre of the culture; cultures derived from cancerous cell lines have obvious disadvantages and normal epithelial cells grown on inserts- to give a more physiological structure- still do not produce an appropriate volume of mucus. The ideal model should recapitulate the exact structure and composition of the native mucus and Sontheimer-Phelps’ Colon Chip goes some way toward doing this.
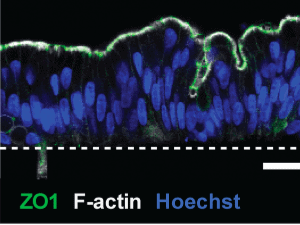
Human colonic epithelial cells were cultured as organoids before being transferred to a chip set-up under dynamic flow. Immunofluorescence was used to confirm the appropriate physiology – polarized columnar epithelial cells with apical tight junctions, an F-actin-rich brush border and basolateral E-cadherin expression. Additionally, the Colon Chip cultures displayed spontaneous goblet cell differentiation- resulting in around 15% goblet cells- equivalent to human colon ex vivo. The authors developed a method to analyse the mucus layer in situ in the Colon Chip – revealing production of a thick mucus layer that replicated the bilayer properties displayed by human colonic mucus in vivo.
Importantly, the authors go on to show that stimulation of the Colon Chip with PGE2 (a potent inflammatory mediator associated with inflammation and raised in ulcerative colitis) led to increased cell proliferation and increased the height of the mucin layer. Further investigation using ion channel blockers indicated that the increase in mucin layer height was occurring via ion and fluid-secretion induced swelling of the pre-existing mucus layer rather than enhanced mucin production. These preliminary data indicate the potential of the Colon Chip as a relevant in vitro tool for evaluation of mucus structure and function, which could advance our understanding of mucus physiology in disease contexts.
This paper also raises an important issue – the ability to access human tissue. The colon models were created using healthy regions of full thickness sections of human colon, obtained during colon resection or biopsies from endoscopic investigations (all with full, informed consent, of course) but it reveals the need for researchers to have access to this valuable resource to enable further development of human-relevant models.
Mucus physiology can be, and has been, studied in vivo in animal models (e.g., using intestinal loop studies) however, these methods are highly invasive, technically challenging, often only low-resolution imaging is possible and they are not done in the species of ultimate interest- the human. With the advances demonstrated in this paper, Sontheimer-Phelps and colleagues offer a dynamic, physiologically accurate, relevant tool that permits live imaging at high resolution and provides insight into disease physiology. The application of specific patient-derived colonic epithelial cells may also facilitate development of new therapeutics or probiotics that modulate the mucus barrier while it provides a novel testbed for personalized medicine.
Image © Sontheimer-Phelps et al., 2019 “Human colon-on-a-chip enables continuous in vitro analysis of colon mucus layer accumulation and physiology” , Cellular and Molecular Gastroenterology and Hepatology made available under Attribution 4.0 International CC BY 4.0.

Post a comment