18 December 2019
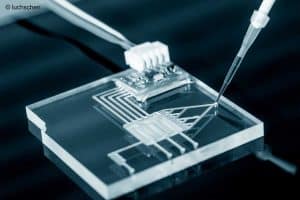
The BioMed21 Collaboration welcomes a recent joint funding call by the South Korean Ministry of Science and ICT and Ministry of Trade, Industry and Energy to develop more human-predictive models for drug assessment. It is well known that there can be poor correlation between toxicity in humans and animals used in laboratory tests, with the latter being predictive of human toxicity only a percentage of the time. This can lead to failures of drugs in the clinic or withdrawal from the market.
This inter-ministerial collaborative project will support 16 RFPs aimed at development and use of human-mimetic methods from research to product development – to better predict the functions of human tissues or organs in vivo – with a view to overcoming the problem of interspecies variability.
This announcement follows a high-level forum in Korea’s National Assembly at which Ministries were invited to discuss proposed new federal legislation that would prioritize funding for human biology-based approaches in biomedical research, and formally establish the Korean Centre for the Validation of Alternative Methods (KoCVAM) under the law with a mandate to work closely with other authorities to advance non-animal method development, acceptance and use. This legislative initiative is spearheaded by BioMed21 Collaboration member Humane Society International and the Korean legal advocacy NGO People for Non-Human Rights, with input from the Korea Legislation Research Institute commissioned by the Ministry of Food and Drug Safety, and National Assembly sponsor Ms. Insoon Nam.

Post a comment