18 May 2020
 by Lindsay Marshall
by Lindsay Marshall
The European Union is on a mission to defeat cancer. This devastating disease disproportionately impacts Europeans, who represent one tenth of the global population, yet comprise one quarter of all cancer diagnoses worldwide. It is no surprise then, that cancer is one of the five mission areas of the EU’s most recent research and innovation framework programme, Horizon Europe. The goal of the mission area focused on cancer is to forestall the staggering number of cancer diagnoses, projected to increase to more than 4.3 million newly diagnosed cases by 2035.
The BioMed21 Collaboration’s team of experienced toxicologists and biomedical scientists, with varied backgrounds in computer modelling, read-across analysis, molecular biology and cellular physiology, has called on the Cancer Mission board to prioritise funding and build capacity for innovative, human-focused, animal-free research and testing strategies that enable the development of precise and reliable models for understanding cancer. We are keen to ensure that the mission adopts a modern research paradigm to enable effective research and development that advances public health through more judicious use of resources. If Horizon Europe’s Cancer Mission is to be a model for effective science, research funding must focus on computational approaches and sophisticated human cell-based models, such as patient-derived organoids, and other innovative, human-relevant methodologies that can better model human cancer. Focusing research efforts on the ultimate species of interest — the human — will offer a faster route to personalised treatments for people with cancer today and will provide an effective platform for evaluating new treatments.
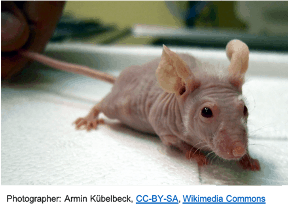 Currently, cancer research maintains a heavy reliance on animals and athymic, nude mice (left) in particular. These
Currently, cancer research maintains a heavy reliance on animals and athymic, nude mice (left) in particular. These 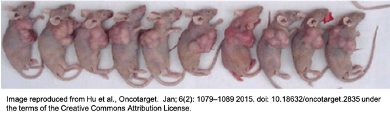 animals lack a fully functioning immune system, facilitating their use as incubators for human tumours (right). This is in contrast to recent developments, including an increased understanding of the importance of the tumour microenvironment, documented issues with infiltration of mouse stromal cells to human tumours in the animal models, recent research revealing the development of significant changes in the nature of the tumours when human cancers are implanted into animals, and the rising interest in immunotherapies as targeted cancer treatments.
animals lack a fully functioning immune system, facilitating their use as incubators for human tumours (right). This is in contrast to recent developments, including an increased understanding of the importance of the tumour microenvironment, documented issues with infiltration of mouse stromal cells to human tumours in the animal models, recent research revealing the development of significant changes in the nature of the tumours when human cancers are implanted into animals, and the rising interest in immunotherapies as targeted cancer treatments.
Recent statistics published by the European Commission reveal that in 2017, 92% of animals used in cancer research in the EU were mice, totalling nearly 1 million animals. This is despite shockingly poor translation to human use: The largest proportion of drug failures is in cancer, where there is approximately 5% likelihood of approval. This means that 95% of the drugs that seem to offer hope for cancer treatment when tested in mouse models fail to have an impact in the patients who need them most—giving rise to lost hope and early deaths and representing a huge emotional, financial and societal burden.
Cancer is a complex, multi-faceted disease that may be initiated by any one or a mixture of genetic, epigenetic and environmental factors. In terms of chemical carcinogenicity, it is vital to fill knowledge gaps on cancer hazard evaluations by means of mechanistically-based testing strategies, and we have encouraged Horizon Europe’s Cancer Mission to invest in hypothesis-based and mechanistic-driven testing strategies. These options would allow better characterisation of the carcinogenic potential of environmental chemicals, are potentially faster than (animal-based) methods currently in use, and would enable authorities to take swifter and more appropriate measures to limit Europeans’ exposure to harmful chemicals that could lead to cancer.
These human-relevant research strategies already exist. A science-driven strategy could conceivably consist of a combination of in vitro methods, ‘omics technologies, and computational approaches, anchored in knowledge captured within a cancer Adverse Outcome Pathway (AOP) or through AOP cancer-networks. The use of human tissues, human-relevant cells and sophisticated human cell-based models, such as the patient-derived organoids, also have the potential to vastly improve the predictive nature of cancer models and offer a route to effective and personalised treatments. We have the tools, we have the technology, and yet sadly, we still have people suffering and dying. We need brave redirection of funding toward more appropriate research methods. The BioMed21 Collaboration calls on Horizon Europe to be a true flagship for innovative science by moving away from a reliance on mice and moving toward a modern, human-relevant research paradigm.
What do you think? How can we improve cancer and make translational research more effective whilst maintaining human-relevance? Share your thoughts in the comments below.

Tuli says
Its high time to develop a tumor model relevant to human rather than killing numerous animals to justify our failure. Heterotypic multicellular structures should be in the frontline.
Lindsay Marshall says
Dear Tuli, Thanks for your input and yes, we totally agree and feel that that dedicating time, effort, funding and innovative approaches to developing better more complex cell-based models are a much better use of resources. We hope that Horizon Europe can also appreciate the need for relevant models and human-centric strategies and will word their funding calls appropriately. Best, Lindsay.