Toward a human-focus paradigm in health research
We organize and sponsor international workshops to facilitate one of the key missions of the BioMed21 collaboration – to bring relevant players together in order to identify opportunities, acknowledge existing barriers, and make recommendations necessary for implementation of a human biology platform for biomedical research. Here you can learn about our events: the speakers, their presentations, and formal accounts of the workshops, including our published workshop reports.


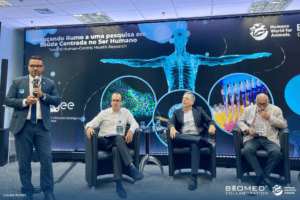

The final workshop in our series took place in Brasília, Brazil, on March 13, 2025—the successful conclusion to our events following Brussels and New Delhi. Organized by #BioMed founding partner, Humane World for Animals (formerly Humane Society International), and Centro de Gestão e Estudos Estratégicos (CGEE), the workshop brought together leading scientists, funding agencies, start-ups, scientific societies (SBPC, ABC), and key agencies like Renama – Rede Nacional de Métodos Alternativos (Brazilian National Network of Alternative Methods).
This workshop series has exceeded expectations, catalyzing policy momentum and scientific collaboration across regions! A full report on these discussions will be published later in 2025. We remain committed to advancing human-centric non-animal methods in biomedical research—whether through government-sponsored projects in India, policy development in the EU, or NAMs integration in Brazil’s National Strategy for Science and Technology. Thank you to LUSH for providing financial support
Key Outcomes:
- Strong calls from scientists for major investment in non-animal methods (NAMs) in Brazil
- Inclusion of NAMs in CGEE’s “purple book” on the 5th National Strategy for Science and Technology, paving the way for national policy integration
- Discussions on possible joint funding calls between Brazil’s state agencies and the Ministry of Science and Technology, with strong Ministry of Health support
- Proposals to strengthen Renama, broadening its scope and designating specialist labs for NAMs
- Offers of collaboration from the European Commission through EU funding instruments, including the European Research Council (ERC) and the Marie Skłodowska-Curie Actions




The BioMed21 Humane World for Animals India team hosted our second science policy workshop in Delhi, Inida on December 8th, 2025. This event, in collaboration with the Biotechnology Industry Research Assistance Council (BIRAC) and the Office of the Principal Scientific Adviser to the Government of India, aimed to explore strategies for promoting a shift toward greater adoption and funding of human-centric, nonanimal methodologies in biomedical research.
We were pleased to welcome a diverse group of experts representing funding agencies, biotech and pharma, academia and public health—united in our shared goal of driving greater adoption of human-centric nonanimal methodologies in biomedical research. By fostering collaboration and highlighting innovative funding mechanisms we aim to pave the way for transformative, human-centric research strategies.
Thank you to LUSH for providing financial support.





On November 18, 2024, we hosted our inaugural science policy workshop in Brussels, Belgium, bringing together leading global experts to rethink how animal research is funded and conducted in the EU. This diverse group included top scientists, policymakers, funders, and research leaders, all at the forefront of biomedical innovation. Together, we explored how human-centric nonanimal research strategies can be harnessed to address urgent public health issues and discussed ways to influence the next EU framework programme.
Focus areas:
– Reimagining biomedical R&D with non-animal, human-centric technologies
– Reassessing research infrastructure to ensure future-proof solutions
– Driving science-based, transformative policy changes
Thank you to LUSH for providing financial support.
A report from the workshop titled, “Leveraging innovative research tools to meet public health challenges”, has been published in the NAM Journal. This report captures a consensus among international experts: to truly advance public health, we must accelerate the shift toward human-centric, multidisciplinary research and embrace innovative, animal-free methods. The recommendations aim to inform EU research funding programs and call for transformative policy and funding reforms.
The workshop and this resulting report offer a clear roadmap for shifting toward human-centric, multidisciplinary, and sustainable biomedical research strategies that truly serve public health, while taking ethics and animal welfare into account.


On 24 April, 2024, BioMed21 partners, Humane Society International, Centre for Predictive Human Model Systems – CPHMS and STEMPeers, hosted a virtual webinar exploring the role of harmonised standards as a first step in building confidence in MPS, and how public and private funding programs can promote the development and use of these systems.
Microphysiological systems (MPS) are capable of simulating critical features of human organ systems and have demonstrated great potential in revolutionizing drug testing and development. Despite this, barriers remain to the adoption of MPS across the globe. In the first part of the session, Dr Lorna Ewart (Emulate) and Dr Abhijit Majumder (Indian Institute of Technology, Bombay) lead discussions on the standardisation of microphysiological systems, taking the audience through case study examples of this process for their liver-on-a-chip and placenta-on-a-chip systems.This was followed by a panel discussion with regulators, government stakeholders, and private funding bodies on how dedicated funding programs can accelerate the development of MPS.
Session Chair:
Dr Y K Gupta, Chair Technical Committee Good Lab Practices; Vice Chair National Standing Committee on Medicine & Healthcare Products; President AIIMS Jammu, ex Dir CSIR-IIT
Panel members:
- Dr Sonja Beken (Belgian Federal Agency for Medicines and Health Products (FAMHP)
- Ms Rajeshwari Adheseshan (Bill and Melinda Gates Foundation)
- Dr Glynn Stacey (Director, International Stem Cell Banking Initiative)
- Mr. Vishal Gandhi (CEO, BIORx- Venture Capitalist)
- Dr Dhiraj Kumar (Chief Manager (Health Care) at Biotechnology Industry Research Assistance Council, Department of Biotechnology, Govt. of India)


The series was co-organized by CPHMS and Humane Society International, co-founder of the BioMed21 Collaboration.
Select the links below to view session videos:
- Part 1: Microphysiological Systems
- Part 2: Systems Biology & Pharmacology
- Part 3: Computational Tools & Biological Networks
- Part 4: Understanding Cancer Using Microphysiological Systems
- Part 5: Virtual Demonstration of Organ-on-Chip / Understanding Polycystic Kidney Disease Using Kidney-on-Chip
- Part 6: Use of In-vitro Human Surrogate Models – An Industry Perspective
- Part 7: Use of Human Clinical Samples to build Microphysiological System Models
- Part 8: Tissue Engineering and MPS models
- Part 9: Brain Organoids
- Part 10: In Vitro 3D Osteochondral Model for Drug Screening
- Part 11: In vitro humanized disease models: Focus on 3D bioprinted lung model
- Part 12: The How’s and Why’s of creating human organs in the lab: A step towards accelerating pre-clinical research
- Part 13: Journey of a cell biologist – Exploring signaling pathways to making tissue organoids for insights into human development
- Part 15: A peek into the drug regulatory system
- Part 16: Can biology be a predictive science?
Organized by the National Assembly and Humane Society International/Korea, this biomedical research multi-stakeholder forum was held in Seoul, So. Korea.
Agenda (English
Agenda (Korean)
Press Release (English)
Video (English- Full Length)
Video (English – 1 min)
Video (Korean – Full Length)

Microphysiological systems – from scientific models towards industrial adaptation of qualified assays and their regulatory acceptance
– Uwe Marx, CSO & Founder, TissUse, Germany
U.S. interagency OOC development program: from funding to qualification for regulatory acceptance
– Suzanne Fitzpatrick, FDA, United States
Introducing new legislative effort, Act on the promotion of development, distribution and use of alternatives to animal testing
– Borami Seo, Interim ED, Senior Policy Manager, Humane Society International, Korea
Industry’s role for OOC development and regulatory applications
– Adrian Roth, Roche, Switzerland
The Forum was organized by the National Assembly in Seoul, South Korea

National Assembly, Seoul, South Korea

Jamia Hamdard, New Delhi, India

Convened in Guangzhou, China.
Humane Society International, co-founder of the BioMed21 Collaboration, was a founding co-organizer and sponsor of the Asian Congress on Alternatives and of the annual Chinese Conferences on Toxicological Alternatives and Translational Toxicology.

Convened in Shanghai, China’s 8th Workshop on Alternative Methods was co-sponsored by Humane Society International, co-founder of the BioMed21 Collaboration.

National Institutes of Health, Bethesda, Maryland, United States.

D’Or Institute, Rio de Janeiro, Brazil

Brussels, Belgium

Share this:
- Click to share on X (Opens in new window) X
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on LinkedIn (Opens in new window) LinkedIn
- Click to email a link to a friend (Opens in new window) Email
- Click to print (Opens in new window) Print
- More
- Click to share on Reddit (Opens in new window) Reddit
- Click to share on Tumblr (Opens in new window) Tumblr
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Pocket (Opens in new window) Pocket
- Click to share on Telegram (Opens in new window) Telegram
- Click to share on WhatsApp (Opens in new window) WhatsApp


